Research background
Normal excitation of the heart and cardiac arrhythmia
Cardiac arrhythmias are the leading cause of death in the Western world. The management ofcardiac arrhythmias and related diseases currently accounts for about 9% of the total health-care expenditure across the European Union. In order to understand the mechanism of cardiacarrhythmia, we will first explain how the normal heart works, see Fig 1.
The heart consists of 4 parts: 2 upper chambers, called the atria, and two lower chambers, the ventricles. The contraction of the heart is initiated by an electrical wave which automatically starts at the sinoatrial nodein the right atrium. From there, the electrical wave spreads through the whole right and left atrium. The electrical signal is then delayed at the AV node, before it spreads rapidly through the bundle of his and excites both ventricles. As the electrical wave initiates contraction, the atria contract first, pumping the blood into the ventricles, after which the ventricles contract, pumping the blood into the lungs and the whole body.
A normal rhythm of the heart results in about 60 beats per minute, which accumulate to about 3 billion beats in a lifetime. One can imagine thatit is possible for this electrical wave to be disturbed, resulting in cardiac arrhythmia.
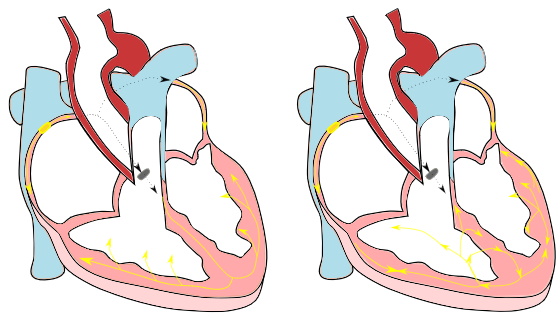 Fig 1. Normal excitation (on the left). The yellow dot indicates the sinoatrial node and the gray dot represents the AV-node. On the right, disturbed excitation leading to cardiac arrhythmia
Fig 1. Normal excitation (on the left). The yellow dot indicates the sinoatrial node and the gray dot represents the AV-node. On the right, disturbed excitation leading to cardiac arrhythmiaThere are two main driving mechanisms for cardiac arrhythmias: reentry and triggered activity. Anatomical reentry occurs when the electrical wave starts to rotate around an obstacle, e.g. around a valve, a vein or scar tissue, which can be compared with a Mexican wave. This rotation sustains itself and is usually very fast, taking over the normal rhythm of the heart of the sinoatrial node. Depending on the size of the smallest rotation, one can make a distinction between macro.
reentry (large obstacle) or localized reentry (small obstacle). However, reentry is also possible without an obstacle, whereby the wave rotates around an excitable core, which is called functional reentry. Functional reentry is also often denoted a spiral wave or a rotor. The second driving source is triggered activity or automaticity. The latter mechanism gives rise to focal activation, with most likely a concentric activation pattern. One can view this as extra sinoatrial nodes which occur at wrong places in the heart, and if fast, they can also take over the normal rhythm.
We can divide most arrhythmia in two different types: the regular arrhythmia, called tachycardia, and the irregular arrhythmia, called fibrillation as they make the atrial of ventricular walls fibrillate. Therefore, including the location of the arrhythmia, we distinguish 4 main types of arrhythmia: atrial tachycardia (AT), atrial fibrillation (AF), ventricular tachycardia (VT) and ventricular fibrillation (VF). A tachycardia can have two mechanisms, a regular reentry or a regular single focal source, while fibrillation can be any combination of multiple irregular sources, reentries and focal sources which appear irregularly in the heart.
Treatment of cardiac arrhythmia
It is the task of a cardiac electrophysiologist (EP) to determine the mechanism of a cardiac arrhythmia. In case medication is not successful, the EP can perform ablation therapy in order to stop the arrhythmia.
 Fig 2. Ablation of a patient
Fig 2. Ablation of a patientFor this, the electrophysiologist (medical doctor) inserts a catheter (measuring electrodes) in the heart and records the electrical activity in the heart. As a result, the medical doctors can obtain a color coded map, as presented in the Figure \ref{map}. In case a clear reentry is detected in the heart, the EP will create a scar which interrupts the reentry path. In case a focal source is detected, this location will be ablated so the focal source can no longer emit electrical activity. It can be very challenging to precisely determine the mechanism of an arrhythmia in a patient. Moreover, wrong ablation lines can make the heart even more prone to new arrhythmia, while also limiting the contractility of the heart. Unfortunately, in the clinical practice there still exist no good automatic algorithms to determine the location of cardiac arrhythmia. This is exactly the problem which we tackle in our research group.
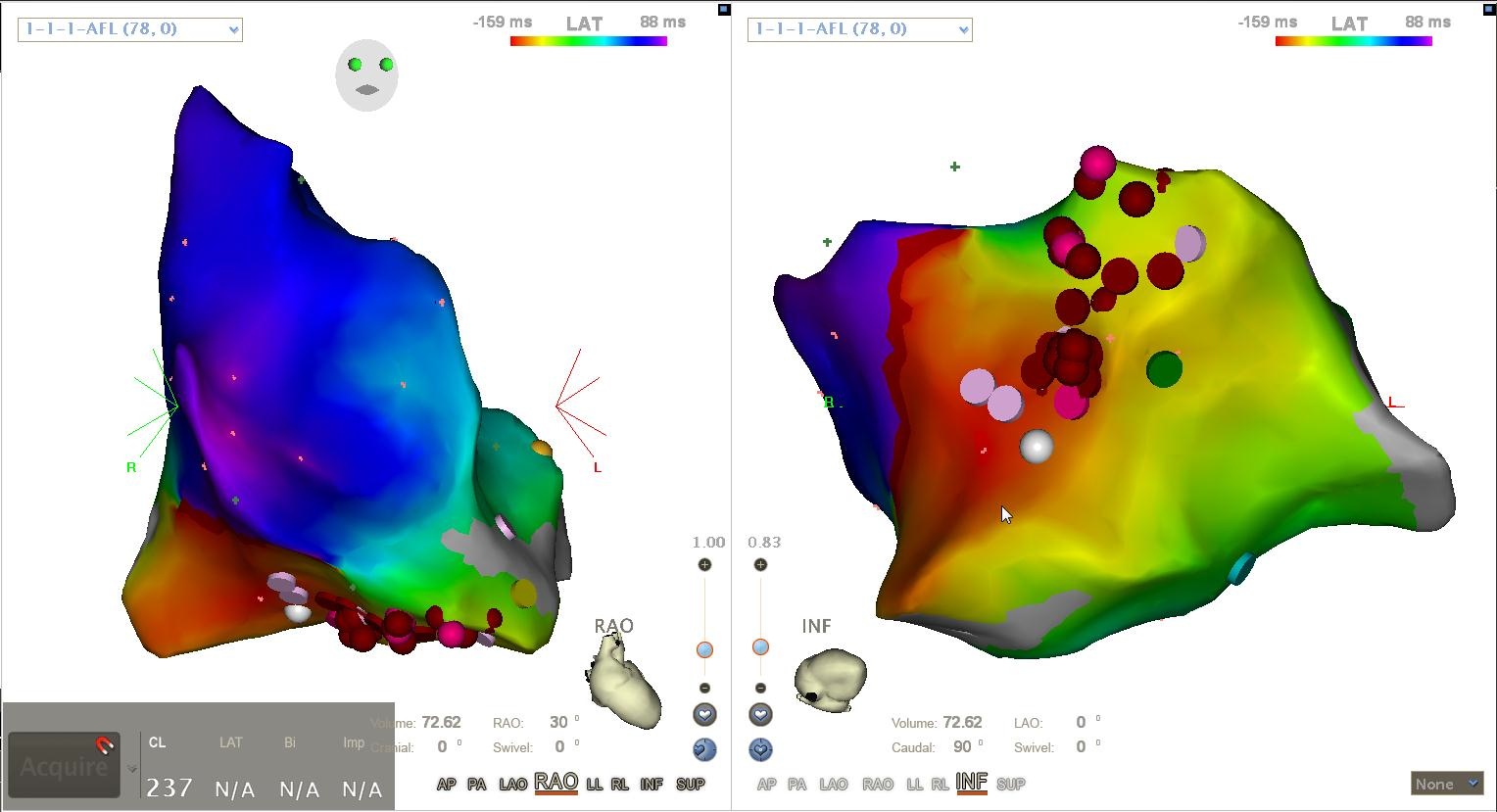 Fig 3. A color coded map created with the mapping system CARTO by Biosense Webster for an atrial tachycardia. The red dots indicate the ablation points
Fig 3. A color coded map created with the mapping system CARTO by Biosense Webster for an atrial tachycardia. The red dots indicate the ablation pointsDirected Graph Mapping - DGM
This map needs to be interpreted by the medical doctor, which requires a high specialism. Interpretation of these catheter maps can be often difficult and prone to interpretation. Different EPs can have a complete different ablation strategy based on the same map. Recently, at Ghent University, we have developed an automatic algorithm which can detect any type of rotational activity or focal activity, which we called directed graph mapping (DGM). For this we have merged concepts of network theory with cardiac arrhythmia. Network theory is used for analysing brain activity, social networks and Google searches to name only a few applications. It has numerous applications in many different fields, but strangely enough it was never applied to the heart, although this is a very natural description of cardiac excitation.
DGM takes as input measurements which have been gathered by the catheter. After the doctor has finished to map the inside of the atria, we can apply our tool (see Figure). The XYZ-coordinates of the electrodes and the Local Activation Time (LAT) (the times when the electrical waves passed in the heart) are loaded in the software. With this information, a directed graph is created. Then we apply special algorithms to find cycles in the network. Afterwards, additional filters are applied (which rates the loops based on how orthogonal they pass the wavefront, we call this phase variance). The software then visualises the rotational circuit or focal source of the arrhythmia, so the doctor can easily know where to ablate. DGM will be patented, and we hope one day to bring our software to the market. However, the are many opportunities to do additional research with this idea, and many our thesis subject are all related to the further development of DGM.
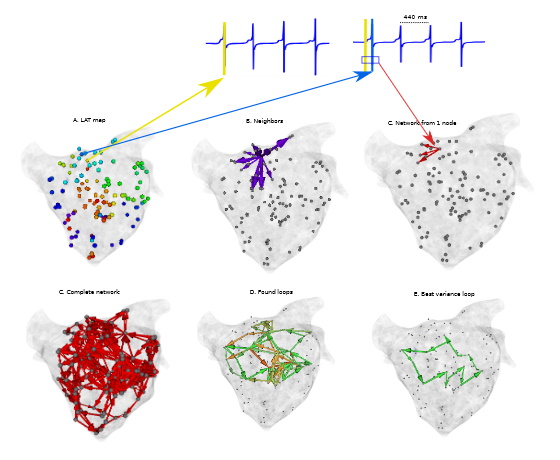 Fig 4. First, the annotated points with corresponding LAT values are loaded (a). Second, for each node, we determine the neighbors and an arrow is drawn in case the difference in LATs values of two neighbors and their corresponding distance is within the considered velocities (b-c). Then, the network is created for each node and merged (d). Afterwards, the cycles are detected (e) and the ones with the highest variance are considered as the responsible of the AT (f)
Fig 4. First, the annotated points with corresponding LAT values are loaded (a). Second, for each node, we determine the neighbors and an arrow is drawn in case the difference in LATs values of two neighbors and their corresponding distance is within the considered velocities (b-c). Then, the network is created for each node and merged (d). Afterwards, the cycles are detected (e) and the ones with the highest variance are considered as the responsible of the AT (f)Computer modeling
Besides DGM, we also focus on computer modeling of the heart. For this we have adopted openCARP, which is a community driven open source code for generating computer simulations of electrical propagation of cardiac excitation. The benefits of using openCARP instead of your own code are the flexibility in changing your model for simulations. Also, you have to write python based scripts for generating your simulations, which can then be shared with the community upon publishing new research findings. Have a look at the website of openCARP (https://opencarp.org/) for further information.
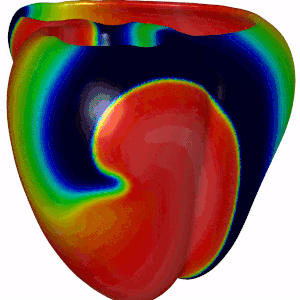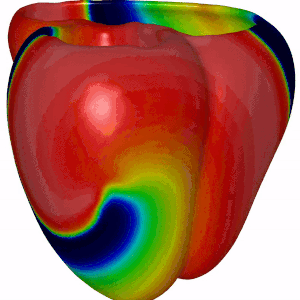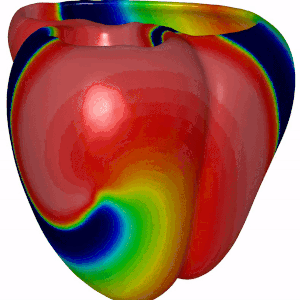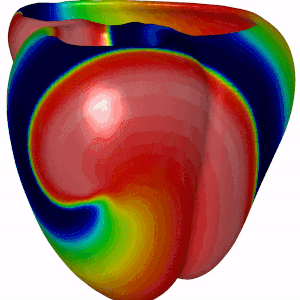 Fig 5. Simulation
Fig 5. Simulation